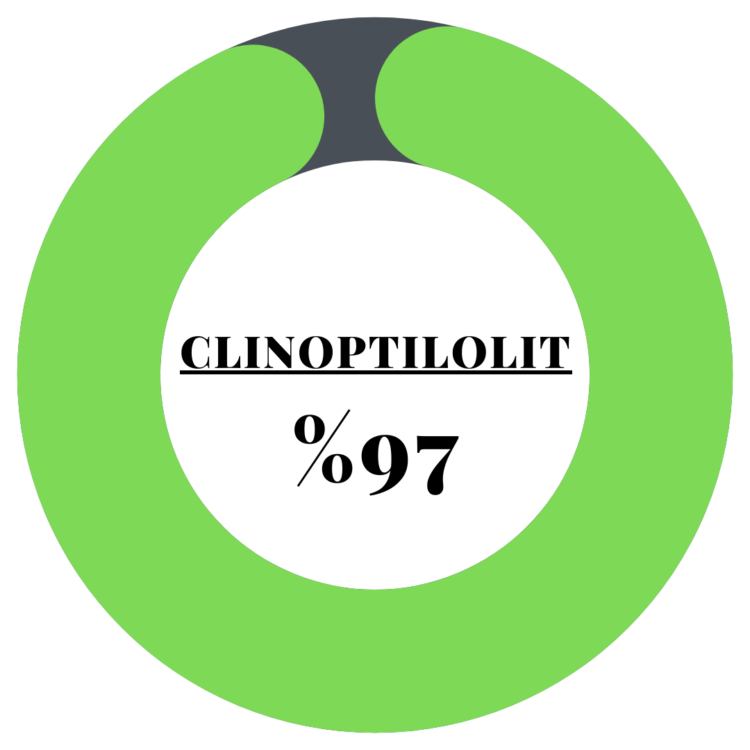
Wastewater Treatment
Nitrogen compounds (especially ammonium) and metal ions (Pb, Cd, Fe, Cu, etc.) present in municipal and industrial wastewater contaminate groundwater and surface water, rendering them unsuitable for both drinking and utility purposes. Additionally, these pollutants have toxic effects on fish and other aquatic fauna, while also inhibiting the growth of algae essential for the ecosystem. Due to their strong adsorption capacity, zeolites can easily capture nitrogen compounds and certain heavy metal cations (such as Pb) from wastewater. In the United States and Japan, many municipal and industrial wastewater treatment plants use clinoptilolite for purification.
Flue Gas Cleaning
Pollutant gases such as CO₂, SO₂, and other emissions released from petroleum- and coal-burning facilities can be controlled using the adsorption properties of zeolites. Studies have demonstrated that mordenite and clinoptilolite are highly effective in this application.